Introduction
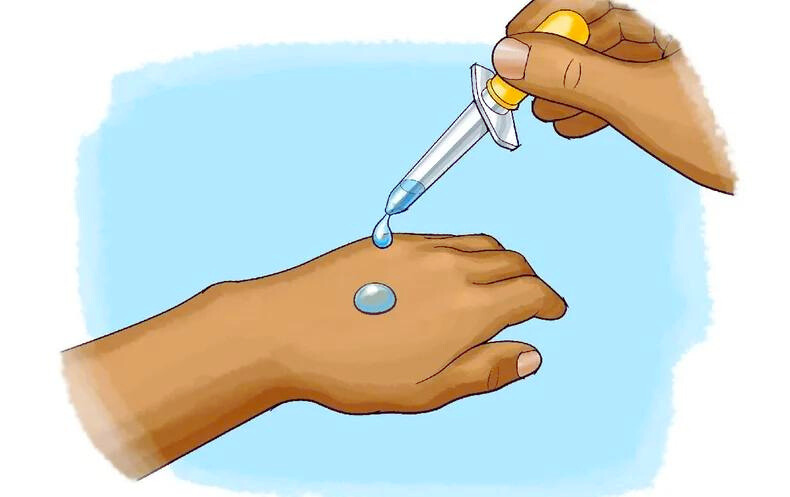
Have you ever wondered why your palm feels unusually cold when you put acetone, petrol, or perfume on it? This intriguing phenomenon has a scientific explanation rooted in the world of chemistry and human physiology. In this article, we’ll delve into the fascinating science behind why certain substances make our palms feel cold and explore the factors that contribute to this unique sensation.
The Chemistry Behind Acetone, Petrol, and Perfume

To understand why acetone, petrol, and perfume cause a cooling sensation, we need to look at their chemical properties. Acetone, a common solvent found in nail polish removers, and petrol, a volatile liquid derived from crude oil, both have low boiling points. Perfume, on the other hand, contains a mixture of volatile aromatic compounds. When these substances come into contact with the skin, they quickly evaporate due to their low boiling points.
Evaporation and Heat Transfer
The key factor behind the cooling sensation is evaporation. When a liquid substance like acetone, petrol, or perfume evaporates, it absorbs heat from its surroundings, including your skin. This absorption of heat leads to a drop in temperature, making your palm feel cold.
Why Do Some Substances Feel Colder Than Others?
Not all liquids produce the same cooling effect. The rate of evaporation depends on the substance’s volatility and the ambient temperature. Substances with lower boiling points evaporate faster, leading to a more noticeable cooling sensation. Acetone, petrol, and certain compounds in perfume evaporate rapidly, intensifying the feeling of coldness on the skin.
How Our Skin Detects Temperature Changes
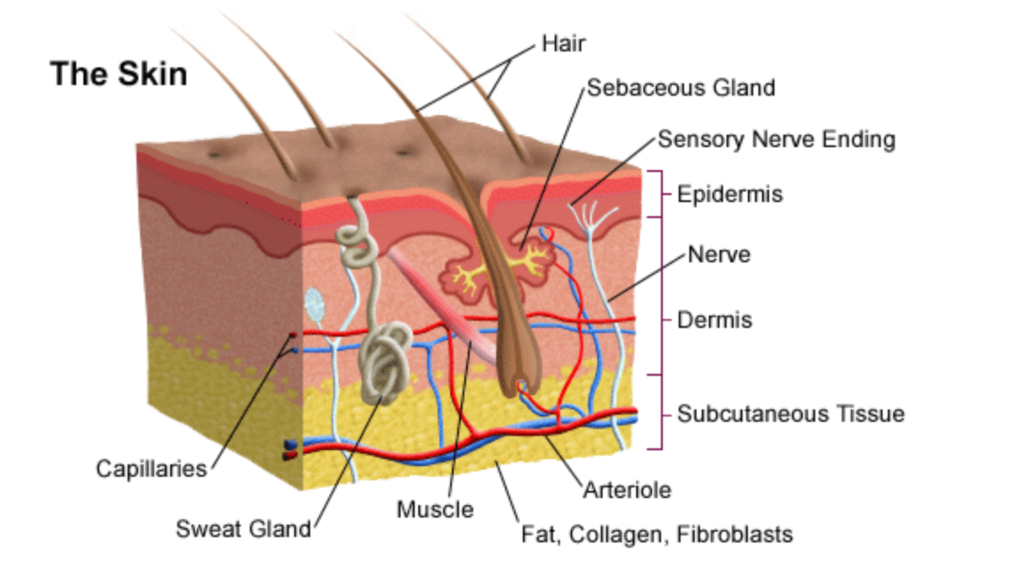
Our skin is equipped with specialized receptors that detect temperature changes. These receptors send signals to the brain, allowing us to perceive sensations such as heat and cold. When acetone, petrol, or perfume evaporates on our skin, these receptors detect the drop in temperature, creating the sensation of coldness.
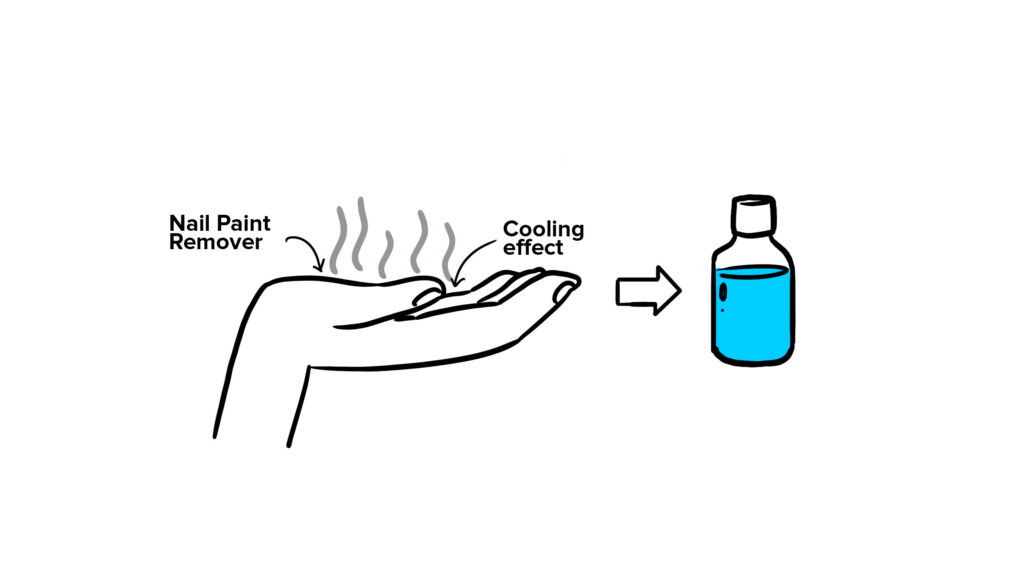
Acetone: A Common Household Chemical
Acetone, commonly used as a nail polish remover, is one of the substances that can make your palm feel cold. Its rapid evaporation rate and ability to absorb heat quickly contribute to the chilly sensation when applied to the skin.
Petrol and Its Cooling Effect
Petrol, a widely used fuel, also produces a cooling effect when it evaporates on the skin. Its volatile nature and low boiling point make it capable of absorbing heat rapidly, causing your palm to feel cold upon contact.
Perfume: More Than Just a Fragrance
Perfume, apart from its pleasant aroma, contains volatile compounds that contribute to the cooling sensation on the skin. These compounds evaporate swiftly, creating a refreshing and cool feeling when applied, enhancing the overall sensory experience.
Why Does the Cold Sensation Fade Away?
As these volatile substances continue to evaporate, the cooling sensation gradually diminishes. This occurs because there’s a limit to how much heat the surrounding skin can provide for evaporation. Once the substance has absorbed all the available heat, the cooling effect subsides.
Safety Measures and Precautions
While these substances create a temporary cooling sensation, it’s crucial to handle them with care. Avoid prolonged or excessive contact with acetone, petrol, or perfume, as they can cause skin irritation. Always use them in well-ventilated areas and follow safety guidelines to prevent any adverse reactions.
Conclusion
The cooling sensation experienced when we put acetone, petrol, or perfume on our palm is a result of rapid evaporation and heat absorption. Understanding the chemistry behind this phenomenon enhances our awareness of the substances we encounter in daily life. Next time you feel that refreshing chill, you’ll know it’s a fascinating interplay of science and sensory perception at work.
Read also: Why Does the Sun Appear Reddish Early in the Morning?
FAQs
Can other substances apart from acetone, petrol, and perfume create a similar cooling effect?
Yes, substances with low boiling points and high volatility can also produce a cooling sensation when they evaporate on the skin. Examples include rubbing alcohol and certain cleaning solvents.
Is the cooling sensation harmful to the skin?
The cooling sensation itself is not harmful, but prolonged or excessive contact with these substances can cause skin irritation. It’s essential to use them in moderation and follow safety precautions.
How long does the cooling sensation typically last?
The duration of the cooling sensation varies based on factors such as the amount of substance applied, ambient temperature, and individual skin characteristics. It usually lasts for a few minutes to half an hour.
Can the cooling effect be used for medical purposes?
In some cases, cooling agents derived from similar principles are used in medical applications, such as topical pain relief gels and patches.
Are there any long-term effects of frequent exposure to these substances?
Prolonged or frequent exposure to acetone, petrol, or perfume can lead to skin dryness and irritation. It’s advisable to use them in moderation and seek medical advice if any adverse reactions occur.