Introduction
In the realm of science, the behavior of water never fails to amaze us. From rainwater trickling down our windows to distilled water used in laboratories, understanding why distilled water doesn’t conduct electricity whereas rainwater does can be intriguing. This article delves into the scientific intricacies, unraveling the secrets behind this phenomenon.
Why Does Distilled Water Not Conduct Electricity Whereas Rainwater Does?
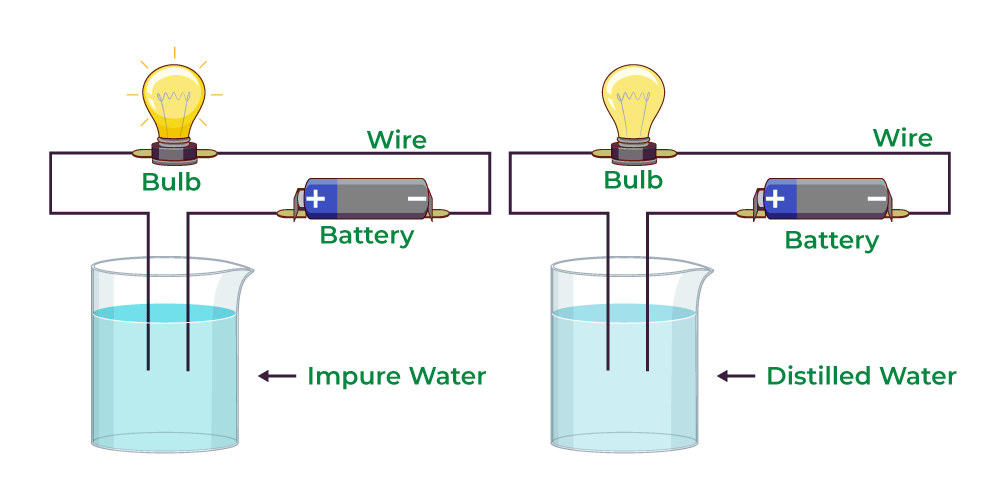
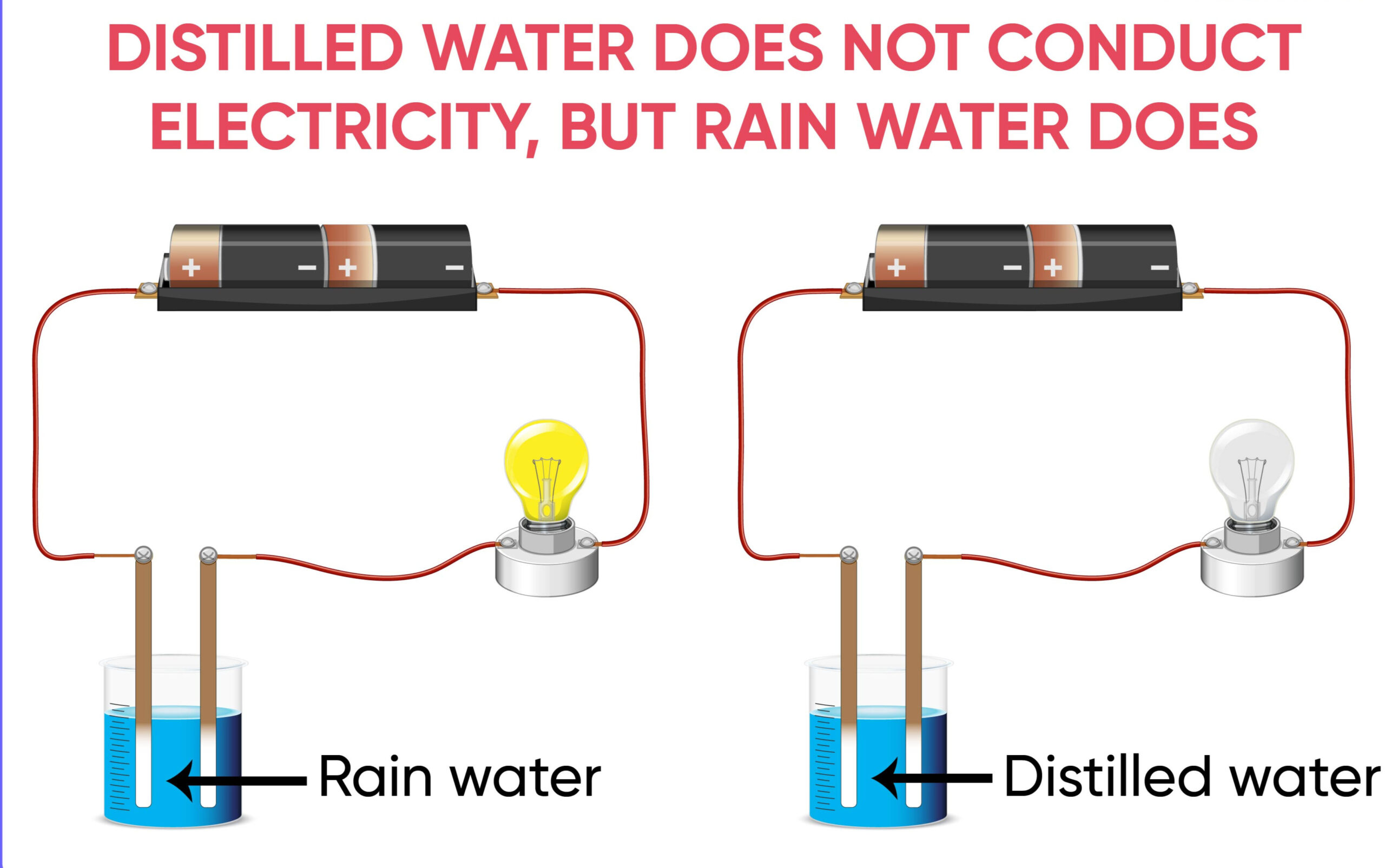
Water, a seemingly simple molecule, exhibits complex behaviors. When it comes to conductivity, the presence of impurities plays a pivotal role. Distilled water, a highly purified form, lacks ions essential for conducting electricity. In contrast, rainwater absorbs various substances while descending through the atmosphere, making it conducive to electricity.
The Role of Ions in Conductivity
Ions: The Conductors of Electricity
Ions are electrically charged particles found in water due to dissolved salts and minerals. Distilled water, purified to remove these ions, lacks the conductive material. Rainwater, however, absorbs ions from the atmosphere and its surroundings, enhancing its conductivity.
Purity of Distilled Water
Distillation: A Process of Purification
Distilled water undergoes a meticulous distillation process, stripping it of impurities. During distillation, water is boiled, and the steam is condensed back into a liquid, leaving behind contaminants. While this purity is ideal for various applications, it renders the water non-conductive.
Atmospheric Absorption in Rainwater
Absorbing Ions from the Environment
As rainwater falls, it absorbs ions from the atmosphere, as well as from surfaces it comes into contact with. These ions transform rainwater into a conductor, allowing the flow of electricity.
Conclusion
In the fascinating world of science, the behavior of water never ceases to amaze. Understanding why distilled water doesn’t conduct electricity while rainwater does unveils the intricate balance between purity and conductivity. The absence of ions in distilled water and the absorption of these ions by rainwater from its environment showcase the wonders of nature. Whether in laboratories or in the natural environment, water’s conductivity remains a captivating topic, reminding us of the complexity within even the simplest of molecules.
Read also: Why Does the World Remember Einstein as a World Citizen?
FAQs About Water Conductivity
Is Distilled Water Safe to Drink?
Absolutely. Despite its non-conductive nature, distilled water is safe to drink as it lacks harmful impurities.
Can Distilled Water Conduct Any Electricity?
In theory, pure distilled water does have a minute ability to conduct electricity due to the presence of trace ions. However, it’s negligible and insufficient for practical applications.
Why Is Rainwater Used in Some Experiments?
Rainwater, enriched with ions, serves as a reliable conductor in certain scientific experiments, ensuring accurate results.
Can Water Purity Affect Electrical Appliances?
Yes, highly purified water like distilled water doesn’t corrode electrical components, making it suitable for specific applications.
Is Rainwater Harvesting Popular Worldwide?
Yes, many regions harness rainwater for various purposes, highlighting its importance beyond just conductivity.
Does Water Conductivity Vary Across Different Sources?
Absolutely. The conductivity of water varies based on its source, with factors like minerals and contaminants influencing its ability to conduct electricity.