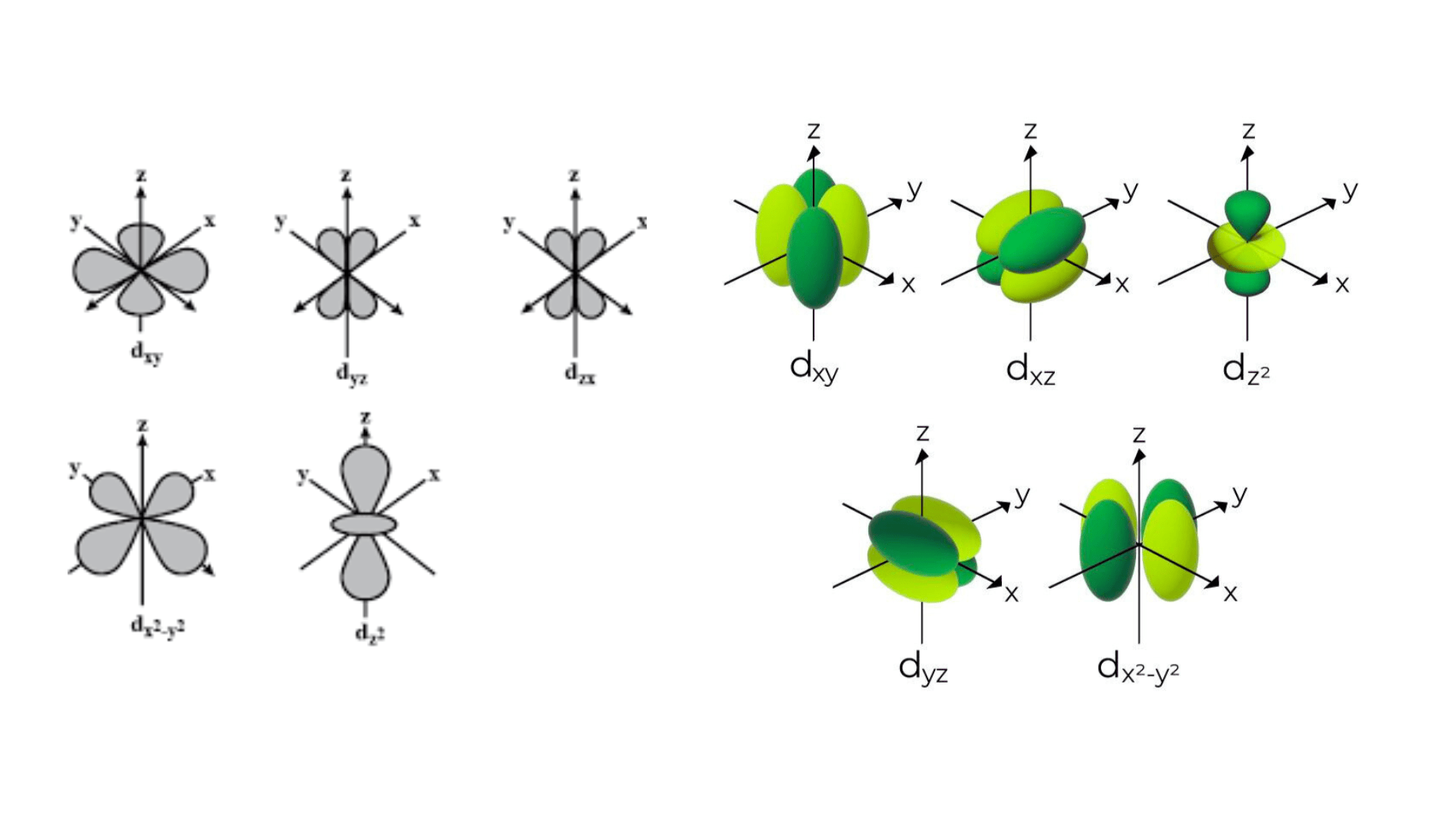
What is an Orbital?
An orbital is a three-dimensional space around an atom where there is a high probability of finding an electron. It can be described by a mathematical function known as a wave function, which details the behavior and location characteristics of electrons within an atom. These descriptions are fundamental to the understanding of atomic theory and quantum mechanics, providing insight into the electron’s wave-like behavior around the nucleus.
Understanding Electron Orbitals
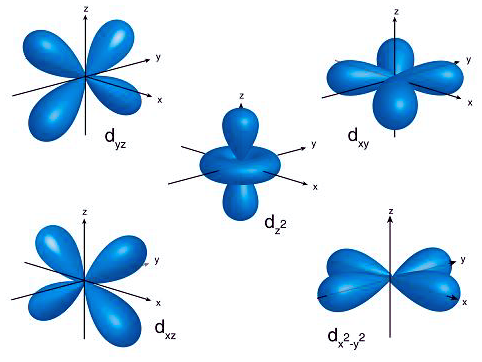
Electron orbitals are essentially mathematical functions that depict the probability of an electron’s presence around an atom’s nucleus. These functions not only predict where an electron is likely to be found but also describe the wave-like behavior of electrons within atoms. Orbitals are categorized into different shapes and energy levels, influencing how atoms interact with each other and form chemical bonds. Each orbital can accommodate up to two electrons, following specific rules such as the Pauli exclusion principle and Hund’s rule, which dictate the filling order of orbitals based on their energy levels.
The Basics of d Orbitals
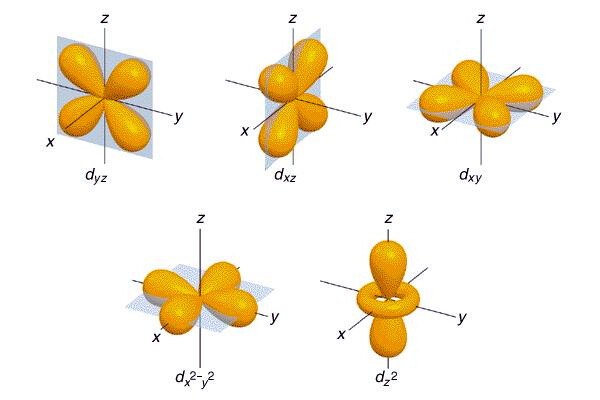
d Orbitals play a crucial role in the chemistry of transition metals, characterized by their complex shapes and involvement in bonding. There are five d-orbitals, designated as dxy, dxz, dyz, dx2-y2, and dz2, each with a unique double dumbbell shape or a cloverleaf shape with varied orientations in three-dimensional space. The magnetic quantum number for these orbitals ranges from -2 to +2, indicating their spatial orientation. d Orbitals become significant in the formation of molecular orbitals, especially in transition metals, where they can combine with other orbitals of compatible symmetry to participate in chemical bonding.
The Shape of d Orbitals
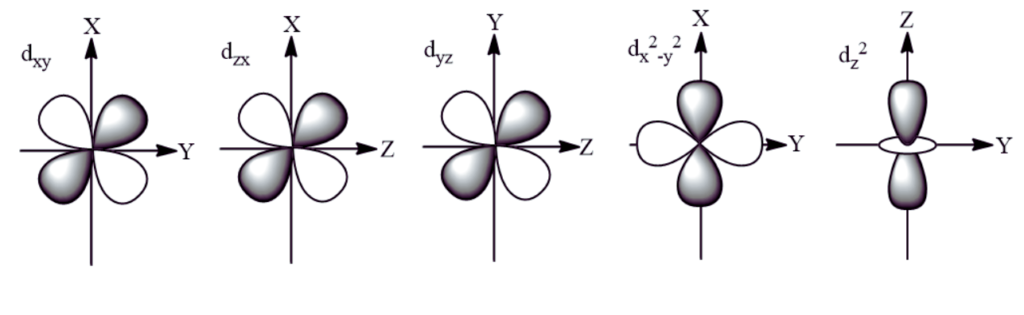
d Orbitals exhibit unique and complex shapes essential for understanding chemical bonding in transition metals. Four of the five d orbitals present a cloverleaf or “daisy-like” shape, characterized by their four lobes extending out in different directions from the nucleus. These orbitals are typically named dxz, dyz, dxy, and dx2-y2, reflecting their orientation in three-dimensional space. The fifth d orbital, dz2, diverges from this pattern, exhibiting a distinctive shape often described as a donut or a dumbbell with a doughnut-shaped ring around the middle. This variety in shapes allows d orbitals to engage in various bonding and hybridization scenarios, playing a critical role in the chemical properties of transition metals.
Types of d Orbitals
There are five distinct types of d orbitals, each with its unique geometry and orientation in space. These orbitals are denoted as dxy, dyz, dxz, dx2-y2, and dz2. The first four orbitals (dxy, dyz, dxz, dx2-y2) have cloverleaf shapes with lobes lying in the plane between the axes indicated by their subscripts. For example, the dxy orbital has lobes lying in the xy-plane. The dz2 orbital is different, featuring a distinctive shape with a doughnut-like ring around the middle, extending along the z-axis. These varied shapes and orientations allow d orbitals to participate in complex bonding patterns, especially in transition metals where these orbitals play a crucial role in chemical reactivity and bonding.
- dxy, dyz, dxz Orbitals
- dx^2-y^2 Orbital
- dz^2 Orbital
dxy, dyz, dxz Orbitals
The dxy, dyz, and dxz orbitals are three of the five d orbitals found in atoms, each with its unique orientation and shape. These orbitals are crucial for understanding the chemical bonding and properties of transition metals.
- dxy Orbital: It is oriented between the x and y axes, with its lobes lying in the xy plane.
- dyz Orbital: This orbital is oriented between the y and z axes, with lobes in the yz plane.
- dxz Orbital: Similar to the dyz, this orbital is oriented between the x and z axes, with its lobes in the xz plane.
Each of these orbitals can form molecular orbitals through overlap with other orbitals, including other d orbitals or orbitals from different atoms. For instance, dxz orbitals can overlap along the z-axis to form a π bond, showcasing the versatility of d orbitals in bonding. Additionally, the arrangement of electrons within these orbitals follows Pauli’s exclusion principle, where each orbital can hold two electrons with opposite spins.
dx^2-y^2 Orbital
The dx^2-y^2 orbital is one of the five d orbitals and is distinguished by its unique orientation and shape. It features lobes that are directed along the Cartesian x and y axes, making it symmetrical along these axes. This orbital is characterized by its lobes lying directly on the x and y axes, as opposed to the dxy orbital, which is rotated 45 degrees and does not align with the axes directly. The dx^2-y^2 orbital’s distinctive configuration is a result of the mathematical solutions to the Schrödinger equation for electrons in atoms, explaining its specific naming and orientation relative to the other d orbitals.
dz^2 Orbital
The dz^2 orbital is distinct among the d orbitals due to its unique shape and electron density distribution. Unlike the other d orbitals which typically have four lobes, the dz^2 orbital comprises two lobes extending along the z-axis and a doughnut-shaped region of high electron density in the xy plane around the nucleus. This configuration results from the orbital’s specific mathematical form as a solution to the Schrödinger equation. The dz^2 orbital is characterized by the presence of conical angular nodes, which separate the doughnut part from the upper and lower lobes, making it appear significantly different from the other d orbitals, which display planar or angular nodal structures.
Visualizing d Orbital Shapes
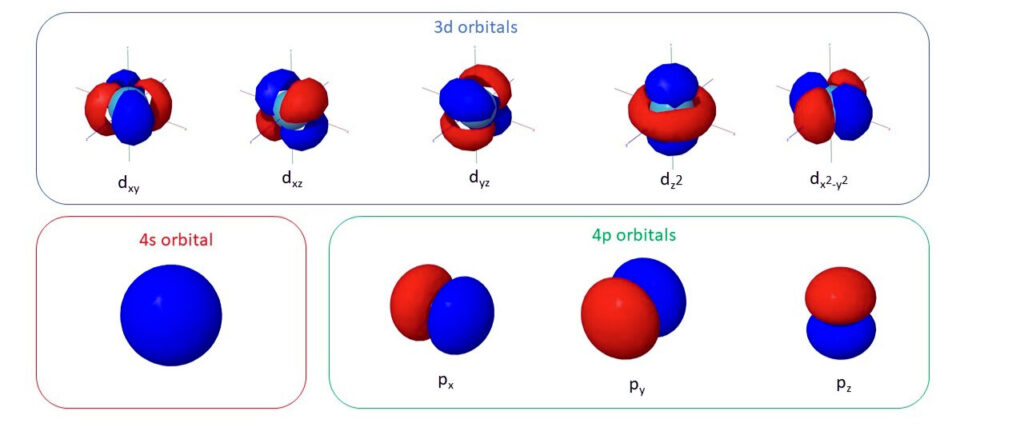
The d orbitals, integral to transition metal chemistry, consist of five distinct shapes that can be visualized in 3D. Four of these orbitals are cloverleaf-shaped, and one (the dz^2 orbital) has a unique shape with two lobes extending along the z-axis and a doughnut-shaped region of high electron density around the nucleus. These orbitals are classified based on their orientation in a rectangular coordinate system, which helps in understanding their spatial distribution and the regions where electrons are likely to be found. The visualization of these orbitals aids in comprehending how orbitals influence chemical bonding and reactivity in transition metals.
The Importance of d Orbitals in Chemistry
d Orbitals play a crucial role in the chemical properties of transition metals, contributing to their unique characteristics. These orbitals are responsible for the variable oxidation states observed in transition metals, a property that is central to their reactivity and the formation of colored compounds. The ability of d electrons to participate in covalent bonding enhances the versatility of transition metals in forming complexes. Moreover, d orbitals are essential in molecular hybridization, expanding the valence shell to accommodate more electrons for bonding, thereby influencing the geometry and bonding capacity of molecules.
The Role of d Orbitals in Bonding
The d orbitals play a significant role in chemical bonding, particularly in transition metals and their compounds. While electrons in s and p orbitals typically form strong bonds, d orbital electrons can form both strong and weak bonds, influencing the chemical properties and reactivity of elements. The d orbitals contribute to the formation of various types of bonds, including sigma (σ), pi (π), and delta (δ) bonds, which are critical for the stability and complexity of chemical structures. They are instrumental in molecular hybridization, expanding the valence shell to accommodate more electrons, thus enabling the formation of complex molecules with various geometries. This flexibility allows for the creation of a wide range of chemical compounds with diverse properties and applications, from catalysis to materials science.
The Role of d Orbitals in Color
The color observed in compounds of transition metals is primarily due to the splitting of d orbitals when metal ions form complexes. In these complexes, the d orbitals split into two energy levels due to the presence of ligands around the central metal ion. When light hits these complexes, electrons can absorb energy and transition between the lower and higher sets of d orbitals. The energy difference between these orbitals corresponds to the energy of visible light. The specific wavelengths (colors) of light absorbed depend on the energy gap between these split d orbitals. The color we see is the complementary color of the light absorbed by these electron transitions. For example, if a complex absorbs light in the violet region, it may appear yellow or orange to the human eye because these are the complementary colors of violet light.
How to Visualize d Orbital Shapes
Visualizing d orbital shapes involves understanding their complex, three-dimensional structures. The d orbitals consist of five types: dxy, dxz, dyz, dx2-y2 and dz2. Each has a unique orientation in three-dimensional space:
- Start with Basic Shapes: The dxy, dxz, and, dyz orbitals resemble four-leaf clovers lying in the xy, xz, and yz planes, respectively. The dx2-y2, orbital also has a clover shape but lies in the x and y axes, orienting along the axes rather than between them.
- Consider the Unique dz2 Orbital: This orbital has a distinctive shape with a doughnut or torus around the middle, extending along the z-axis, and two lobes above and below this torus.
- Use Visualization Tools: Websites like ChemTube3D provide interactive 3D models of the d orbitals, allowing you to rotate and examine their shapes from all angles. Similarly, YouTube tutorials can offer step-by-step guides on drawing these orbitals.
- Understand Nodal Structures: Recognize that d orbitals have nodal planes or surfaces where the probability of finding an electron is zero. This concept is crucial for grasping the spatial orientation and shape of each orbital.
By combining these strategies with resources for visualization, you can develop a more intuitive understanding of d orbital shapes and their significance in chemistry.
Tools and Techniques
Visualizing d orbital shapes can be challenging due to their complex three-dimensional structures. However, several tools and techniques can facilitate this process:
- Drawing on Coordinate Systems: Begin by sketching the x, y, and z axes, then superimpose the d orbitals on this framework to understand their orientation in space.
- Considering Quantum Numbers: Recognize how the three quantum numbers (principal, azimuthal, magnetic) influence the shape and orientation of atomic orbitals, including d orbitals, along the x, y, and z axes.
- Utilizing Online Tools and Software: Explore online tools and software designed for plotting orbitals, which can simplify the visualization process for those without a deep background in physics.
- Video Tutorials: Watch video tutorials that guide you through the process of drawing the shapes of orbitals, providing visual and step-by-step instructions.
- Quantum Chemistry Calculations: Software like Gaussian can calculate and visualize molecular orbitals, offering insights into the delocalized nature of these orbitals.
- Winplot Software: Use Winplot for quick and real-time plotting of hybrid atomic orbitals, facilitating the creation of accurate visual representations.
The Quantum Mechanical Model
The Quantum Mechanical Model, also known as the wave mechanical model, represents the modern understanding of an atom’s structure. It departs from classical models by describing electrons not in fixed orbits but in terms of probabilities and wave functions. This model suggests that the position of electrons around the nucleus is best expressed probabilistically, with electrons found within certain “clouds” or orbitals. Quantum mechanics, the fundamental theory underpinning this model, explains the behavior of particles at and below the scale of atoms and is crucial for understanding the nature of atomic and subatomic particles.
Conclusion
The shape of d orbitals, with their intricate geometry and critical role in the atomic realm, is a fascinating subject that bridges the gap between abstract quantum mechanics and the tangible, colorful world of chemistry. Whether you’re a student, a curious learner, or a seasoned chemist, understanding d orbitals opens up a universe of insights into the behavior of matter at the most fundamental level.
Read also: What Is Verbal Sanitation?
FAQs
Q. How many d orbitals are there?
There are five d orbitals, each with a unique shape and orientation.
Q. Why are d orbitals important?
d orbitals are crucial for understanding the chemical behavior of transition metals, including bonding, magnetism, and color properties.
Q. Can we see d orbitals?
While we can’t see d orbitals with the naked eye, their shapes can be visualized using models, computer simulations, and 3D representations.
Q. How do d orbitals differ from s and p orbitals?
d orbitals have more complex shapes compared to the spherical s orbitals and dumbbell-shaped p orbitals, allowing for a greater variety of chemical interactions.
Q. Are d orbitals relevant in everyday life?
Absolutely! d orbitals play a key role in the color and magnetic properties of many materials we encounter daily, from the pigments in paints to the functioning of electronic devices.