Matter is a fundamental component of the universe, encompassing everything that has mass and occupies space. From the air we breathe to the stars in the sky, matter is everywhere, manifesting in various forms such as solids, liquids, and gases. The properties and behavior of matter can be understood by examining the characteristics of its smallest units: the particles of matter. These particles exhibit several key characteristics:
- Tiny Size: Particles of matter are extremely small, often requiring microscopic or even more advanced techniques to be observed.
- Spaces Between Particles: There are spaces between the particles, which vary in size depending on the state of matter (solid, liquid, gas).
- Constant Motion: Particles are always in motion, with the extent and nature of this motion varying by the state of matter.
- Attraction: Particles of matter attract each other, a force that plays a crucial role in determining the state of matter and its properties.
Understanding these characteristics provides a foundation for explaining why matter exists in different states and how it changes from one state to another, affecting everything from the water cycle to the structure of the universe itself.
The Basics of Matter

Matter constitutes all the physical substances around us; it’s what the universe is made of. Two primary characteristics define matter: mass and volume. Mass is a measure of the amount of matter in an object, often quantified using units such as kilograms or grams. It is a constant property that does not change regardless of the object’s location in the universe. On the other hand, volume refers to the space that an object occupies, which can vary based on the object’s physical state (solid, liquid, or gas) and conditions like temperature and pressure. Together, mass and volume encapsulate the essence of what matter is: anything that occupies space and has mass.
Particle Motion
Particles exhibit different types of motion, including linear motion, vibration, and rotation. Linear motion refers to movement in a straight line, where the direction and magnitude of the velocity are constant unless acted upon by an external force. Vibrational motion involves periodic oscillations about an equilibrium point, such as the back-and-forth movement of a mass attached to a spring. Rotational motion occurs when particles spin around an axis. This can be observed in molecules where atoms rotate around bonds or the molecule itself rotates in space.
Spaces Between Particles
The spaces between particles vary significantly across the three states of matter—solid, liquid, and gas. In solids, particles are closely packed with very little space between them, which accounts for their definite shape and volume. Liquids have particles that are still close but with some spaces between them, allowing for fluidity and the ability to conform to the shape of their container. In the gaseous state, the spaces between particles are the largest, with particles moving freely and randomly. This large spacing accounts for gases’ ability to expand and fill their containers. The variation in spacing is primarily due to the differences in the kinetic energy and forces of attraction between the particles in each state.
Energy in Particles
Particles contain kinetic energy due to their constant motion. The speed at which these particles move dictates the amount of thermal energy present; faster-moving particles indicate higher thermal energy. Energy can be transferred between particles and through space in several ways. In the context of waves, particles can move perpendicular to the wave’s direction, transferring energy via amplitude, wavelength, and frequency. Particles in liquids move rapidly and collide frequently due to the relatively short distances between them, facilitating energy transfer. Thermal energy transfers occur through conduction (direct contact), convection (fluid movement), and radiation (electromagnetic waves). In sound waves, energy transfer occurs through the vibration of air particles or particles within a solid medium. Heat, as a flow of energy, naturally moves from matter containing faster-moving particles to matter with slower-moving particles.
The States of Matter
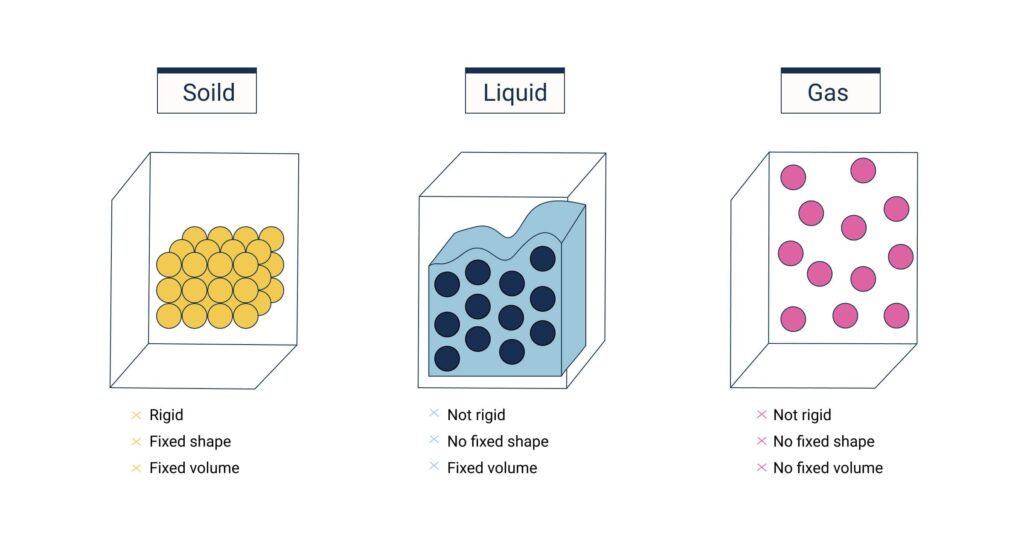
Matter can exist in several distinct states, primarily solid, liquid, gas, and plasma, observable in everyday life. Additionally, Bose-Einstein condensates represent a state achieved under laboratory conditions.
- Solids are characterized by tightly packed particles, giving them a definite shape and volume.
- Liquids have a fixed volume but take the shape of their container, as their particles are less tightly packed than in solids but more so than in gases.
- Gases are composed of particles that are far apart, allowing them to fill the volume of their container fully.
- Plasma, found in stars, is a state of matter where gases are energized to the point that electrons are separated from nuclei, creating a mixture of charged particles.
- Bose-Einstein Condensates (BECs), formed at temperatures close to absolute zero, represent matter with particles that exhibit quantum phenomena on a macroscopic scale.
Each state has unique properties due to the arrangement and energy of its particles.
Solids
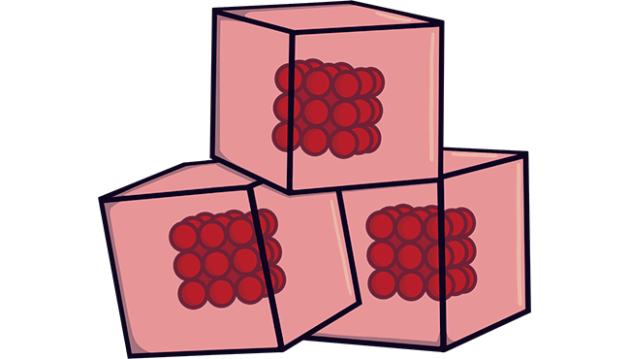
In solid matter, the particles are characterized by a closely packed arrangement and limited movement. The key features include:
- Closely Packed Particles: Particles in solids are tightly packed, often in a regular, three-dimensional array. This close arrangement contributes to solids’ definite shape and volume.
- Limited Movement: While particles in a solid vibrate, they generally cannot move from their positions. This restricted movement is due to the strong intermolecular forces holding the particles in place.
- Definite Shape and Volume: The close packing and limited movement of particles give solids a definite shape and volume. Unlike liquids and gases, solids do not conform to the shape of their container.
These characteristics distinguish solids from other states of matter, such as liquids and gases, where particles are less tightly bound and have more freedom of movement.
Liquids
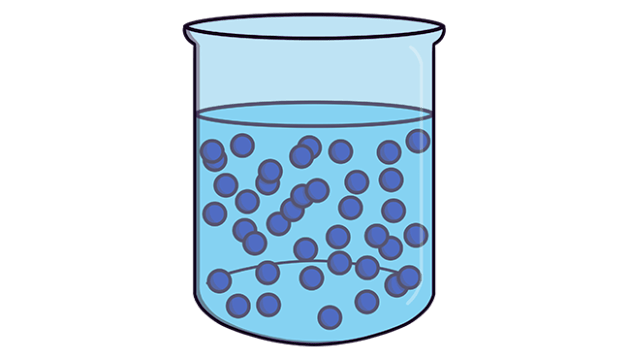
Particles in liquids exhibit a distinct arrangement and movement pattern, characterized by:
- Close Proximity but Irregular Arrangement: Particles in liquids are closely packed but do not have a regular, fixed arrangement. This allows them to slide past each other, facilitating fluidity and the ability to take the shape of their container.
- Vibration and Movement: Liquid particles vibrate and move about, which allows them to slide past each other easily. This movement is more constrained than in gases due to closer proximity to neighboring particles, but it is more free than in solids, where particles are tightly locked in place.
- Mild Force of Attraction: There is a mild force of attraction between particles in a liquid, which is stronger than in gases but weaker than in solids. This intermediate force of attraction allows liquids to flow while maintaining a definite volume.
These properties contribute to the unique behavior of liquids, such as their ability to flow and assume the shape of their container while maintaining a constant volume.
Gases

Gas particles exhibit characteristics distinct from those in solids and liquids, primarily due to their:
- Free Movement: Gas particles are in constant motion, moving rapidly in all directions. This high degree of freedom allows gases to fill any container, irrespective of its shape or size.
- Spacing Between Particles: There is a lot of free space between gas particles, significantly more than in liquids or solids. This large spacing is a key reason gases are easily compressible, allowing their volume to decrease under pressure.
- Collisions: Despite the vast spaces between them, gas particles are constantly colliding with each other and the walls of their container. These collisions are perfectly elastic, meaning there is no net loss of kinetic energy in the system.
These characteristics underline the unique behavior of gases, including their ability to diffuse quickly and occupy the entire volume of their containers.
Plasma

Plasma is often referred to as the fourth state of matter, distinct from the familiar solids, liquids, and gases. It is formed when a gas is heated to very high temperatures or when sufficient energy is applied to strip electrons from atoms, leading to a mixture of free electrons and ions. This ionization process grants plasma its unique properties, including its ability to conduct electricity and respond to electromagnetic fields. Unlike the other states of matter, plasma consists of particles in random motion with long-range electromagnetic forces influencing their interactions. The high-energy state of plasma particles, characterized by their thermal kinetic energy, is typically measured in kelvin or electronvolts, indicating the significant energy levels required to maintain a substance in the plasma state.
Bose-Einstein Condensates
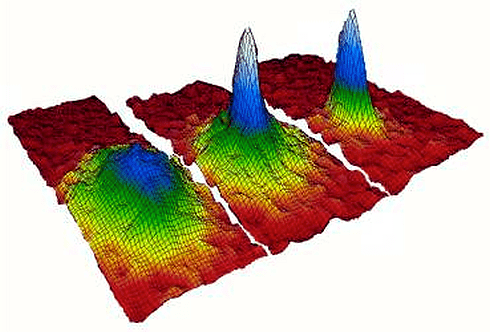
A Bose-Einstein Condensate (BEC) is a state of matter formed under extremely low-temperature conditions, where a group of bosons (particles with integer spin) occupy the same, lowest energy state. This phenomenon leads to the emergence of quantum mechanical properties on a macroscopic scale, such as superfluidity and, in many cases, superconductivity. In a BEC, particles lose their individual identity and behave as a single quantum entity, showcasing a wave-like nature and coherence over macroscopic distances. The formation of a BEC highlights the fundamental principle that particles in this state exhibit behavior governed by quantum mechanics, significantly differing from classical physics predictions.
How Temperature Affects Particle Behavior
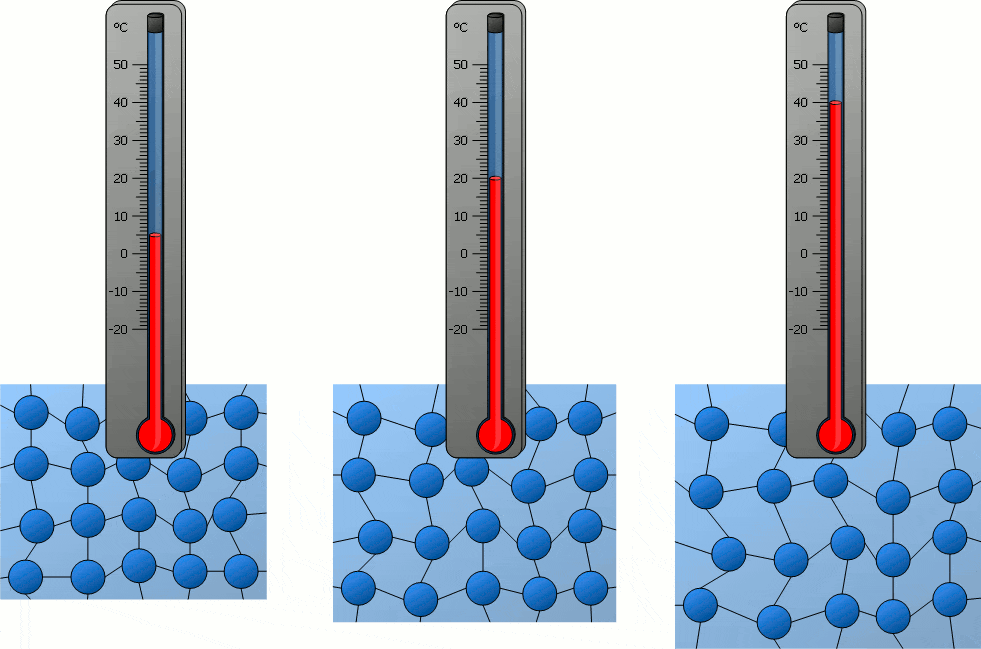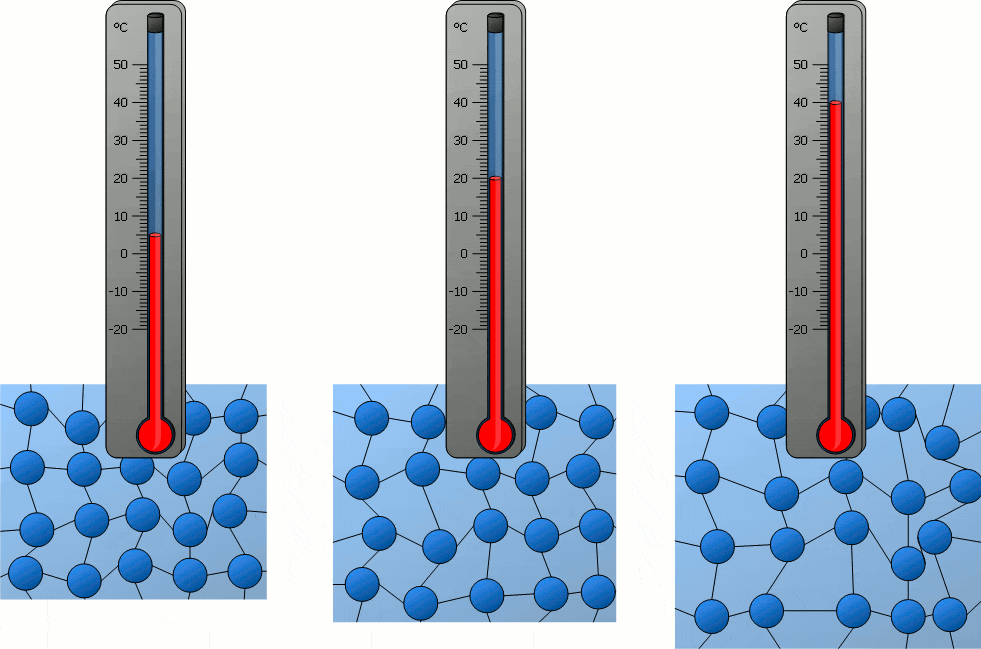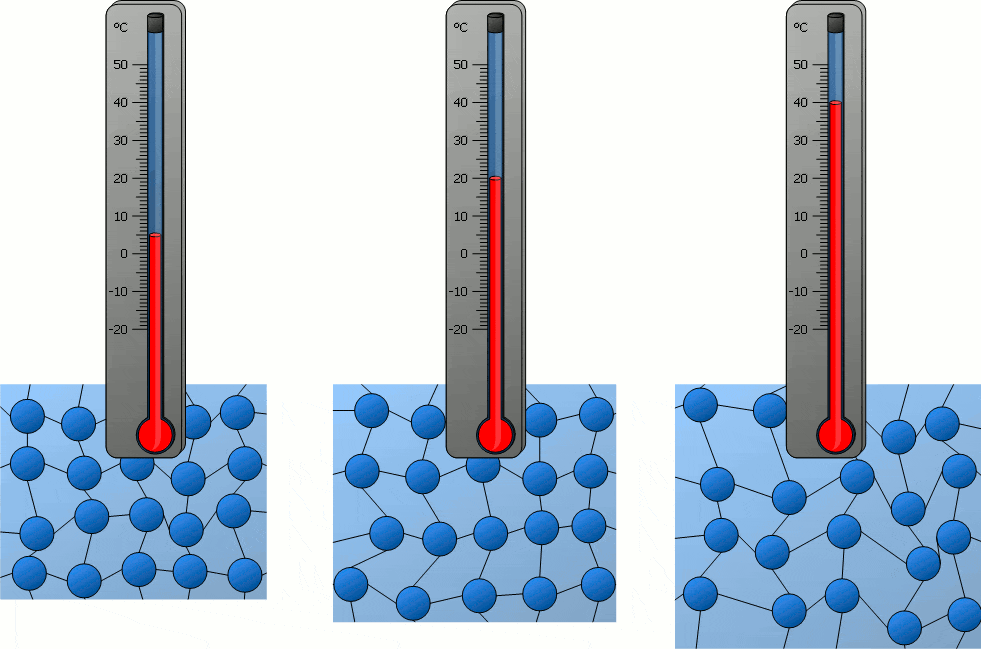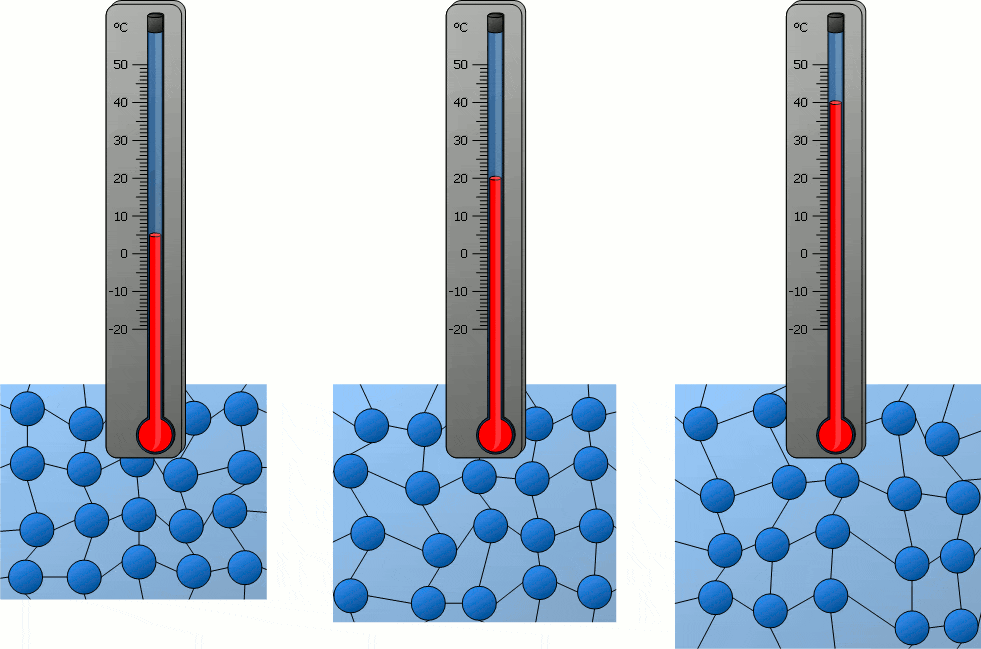
Temperature significantly influences the behavior of particles in matter. As temperature increases, particles gain kinetic energy, causing them to move more rapidly. This can lead to changes in the state of matter, as the increased movement of particles can overcome the forces holding them together in a solid or liquid state. Conversely, decreasing temperature reduces particle movement, potentially solidifying liquids or further solidifying solids. In solids, particles vibrate around fixed positions, with the amplitude of these vibrations increasing with temperature. In liquids, particles move more freely than in solids, and an increase in temperature enhances this mobility. In gases, particles move rapidly in all directions, and higher temperatures lead to faster movement and greater spacing between particles. Therefore, temperature changes directly correlate with changes in particle movement, which can lead to transitions between solid, liquid, and gas states.
Melting and Freezing
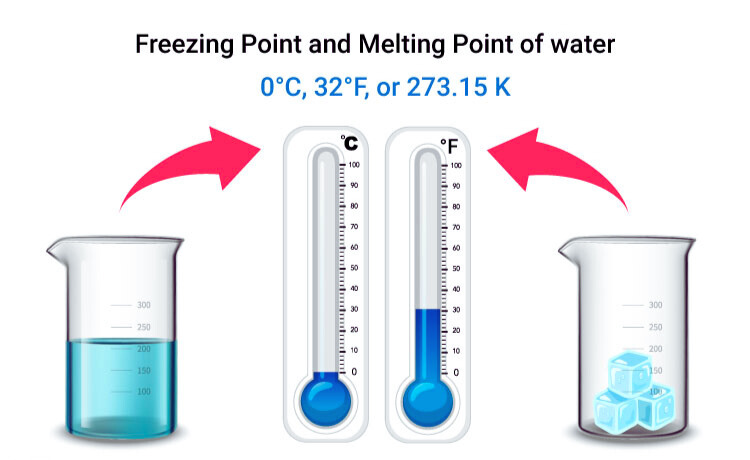
Melting and freezing are processes that describe the transition between solid and liquid states of matter at the particle level.
Melting occurs when a solid is heated. As the temperature increases, particles within the solid gain kinetic energy, causing them to vibrate more vigorously. When the particles’ kinetic energy is sufficient to overcome the forces holding them together in a solid structure, the solid begins to melt, transitioning into a liquid state. During melting, the particles move from a highly ordered structure to a less ordered state, allowing them to move more freely.
Freezing, on the other hand, is the process of changing a liquid into a solid. As a liquid cools, its particles lose kinetic energy and their movement slows down. Eventually, the particles are slow enough for the intermolecular forces to pull them into a more ordered, solid structure. This transition occurs when the particles no longer have enough energy to overcome these forces, resulting in the liquid freezing into a solid.
Both melting and freezing are dependent on the temperature and pressure conditions, with specific substances having characteristic melting/freezing points at which these transitions occur.
Boiling and Condensation
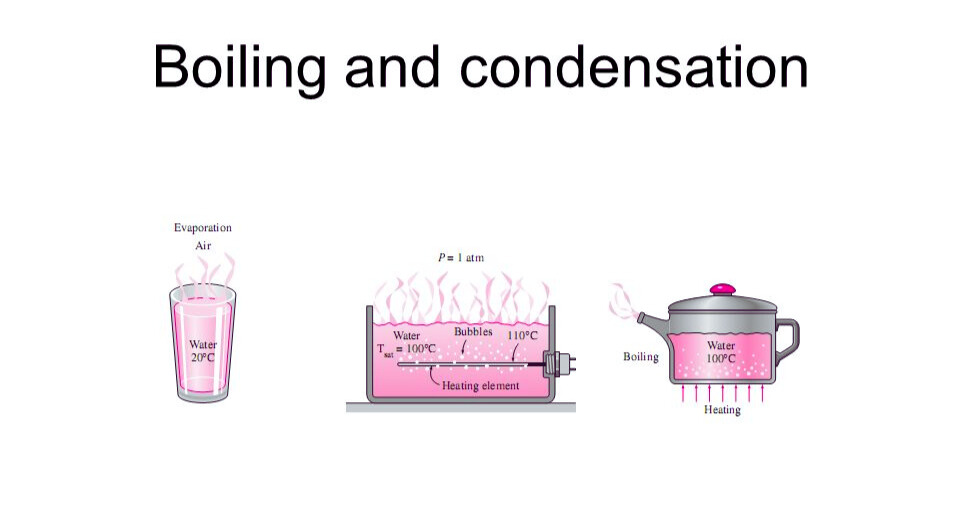
Boiling is the process where a liquid becomes a gas, often referred to as vaporization. During boiling, heat energy is absorbed by the liquid, causing its particles to move faster and eventually overcome the forces holding them together, thus separating and forming a gas.
Condensation, on the other hand, is the process where a gas becomes a liquid. This transformation occurs when the gas particles slow down due to a decrease in temperature or an increase in pressure, allowing the forces between particles to bring them closer together, eventually transitioning into a liquid state.
In summary, boiling involves the separation and rapid movement of particles as they transition from liquid to gas, while condensation involves the slowing, clustering, and transition of gas particles back into a liquid.
Sublimation and Deposition

Sublimation and deposition are fascinating phase changes that involve a direct transition between the solid and gas phases, bypassing the liquid phase. In sublimation, a solid transforms directly into a gas without becoming a liquid first. This occurs when the particles of the solid gain enough energy to overcome their intermolecular forces and disperse into a gaseous state. Common examples include dry ice (solid carbon dioxide) turning into CO2 gas at room temperature.
Deposition, on the other hand, is the reverse process where a gas changes directly into a solid, without passing through the liquid phase. This process can be observed when frost forms on cold surfaces, as water vapor in the air deposits into ice crystals. During deposition, gas particles lose enough thermal energy to allow intermolecular forces to bring them together, forming a solid structure.
Both processes are driven by changes in energy and the ability of particles to overcome or submit to intermolecular forces. They showcase the dynamic nature of matter and its ability to transition between states under different conditions.
Conclusion
Understanding the characteristics of matter’s particles unveils a universe of complexity in the seemingly simple. It’s a reminder of the intricate dance of energy and movement that forms the foundation of everything around us.
Read also: What Are the Functions of Stomata?
FAQs
Q. What determines the state of matter?
The state of matter is primarily determined by the energy of its particles and the forces between them.
Q. Can matter change states?
Yes, matter can change states through processes like melting, freezing, boiling, and condensation, depending on temperature and pressure conditions.
Q. What is the rarest state of matter?
The Bose-Einstein condensate is considered the rarest state of matter, existing only under extreme cooling conditions.
Q. Do all materials have a boiling point?
Most materials have a boiling point at which they transition from liquid to gas, though the temperature can vary widely depending on the substance.
Q. What’s the difference between an atom and a molecule?
An atom is the smallest unit of an element that retains its identity, while a molecule is a group of two or more atoms bonded together.



amazing veray greatetopic nice information amazing fantastik ✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨✨