Introduction: The Marvelous Transformation of Carbon into Diamonds
Diamonds have captivated humans for centuries with their dazzling beauty and timeless allure. But have you ever wondered how these precious gems, adorning the most exquisite jewelry, are made? The process of diamond formation is as intriguing as the gems themselves, involving immense pressure, heat, and a dash of magic from Mother Nature. In this article, we will delve deep into the mysterious world of diamond formation, exploring the intricate journey from carbon to brilliance.
Understanding the Basic Building Blocks: Carbon Atoms

At the heart of every diamond lies the humble carbon atom. These atoms, arranged in a crystal lattice structure, undergo a remarkable transformation to create the hardest known substance on Earth. To comprehend this transformation, we need to explore the conditions under which diamonds are born.
The Deep Earth’s Kitchen: High Pressure and High Temperature

Diamonds are formed deep within the Earth’s mantle, under conditions of extreme pressure and temperature. Imagine a depth of 140 to 190 kilometers beneath the Earth’s surface, where carbon atoms are subjected to pressures reaching around 725,000 pounds per square inch and temperatures soaring above 2,200 degrees Fahrenheit. It’s under these intense conditions that carbon atoms start their magical journey toward becoming diamonds.
Magmatic Marvels: Diamonds in Volcanic Pipes
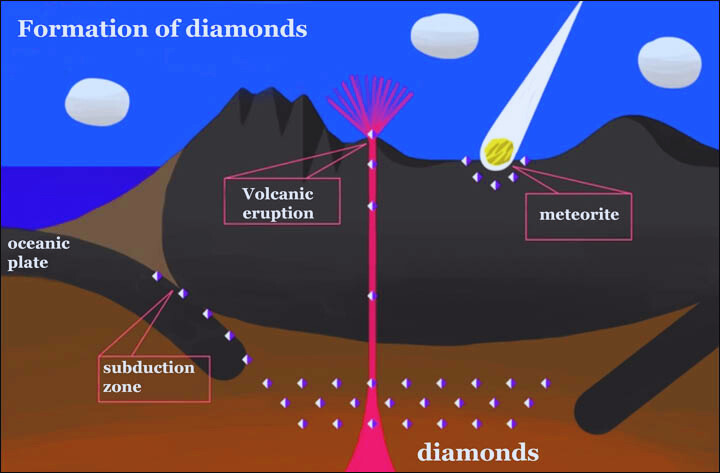
The transformation of carbon into diamonds often begins in the molten rock, or magma found deep within the Earth. This magma, laden with carbon-rich materials, rises through volcanic pipes, carrying the raw ingredients for diamond formation towards the Earth’s surface. As the magma cools and solidifies, it creates unique structures called kimberlite pipes, which sometimes contain diamonds.
Crystallization: From Carbon Soup to Diamond Crystals

Within these kimberlite pipes, carbon atoms slowly crystallize to form diamond structures. The process is incredibly slow, taking millions to billions of years. During this time, carbon atoms align perfectly in a three-dimensional structure, creating the mesmerizing brilliance that diamonds are known for. The specific conditions during this crystallization process give diamonds their varied colors and unique inclusions, making each diamond a distinct masterpiece.
Human Intervention: Mining and Cutting Diamonds

While nature has its way of creating diamonds, humans have found methods to extract these precious gems for various purposes. Mining operations, often located near diamond-rich geological formations, extract diamonds from the Earth’s crust. Skilled artisans then cut and polish these rough diamonds, enhancing their natural beauty and preparing them for their journey into the world of jewelry and luxury.
Conclusion: A Symphony of Time, Pressure, and Beauty
In conclusion, the journey from carbon to diamond is a symphony of time, pressure, and beauty. Nature, with its immense power and patience, crafts these gems that continue to enchant and inspire us. The next time you gaze upon a diamond, remember the remarkable journey it undertook, from the depths of the Earth to the brilliance adorning your jewelry.
Read also: How Are You in French: A Comprehensive Guide to French
The natural process of diamond formation takes millions to billions of years under extreme pressure and temperature conditions deep within the Earth’s mantle.
Yes, scientists can create diamonds in a laboratory setting using high-pressure, high-temperature (HPHT) or chemical vapor deposition (CVD) methods.
Diamonds are expensive due to their rarity, beauty, and the intricate process involved in their formation. High demand and limited supply also contribute to their high value.
No, diamonds come in various colors, including yellow, brown, blue, green, and even rare colors like red and pink. These colors are caused by trace elements or structural defects in the crystal lattice.